New drug approval license of Mitoxantrone hydrochloride injection for tracing;
Gosun's cefazolin and ceftazidime for injection won bidding in National centralized procurement;

Started the construction of the first manufacture factory for Gosun.

Gosun's synthesis workshop was completed and put into production.

Became Provincial GMP system pilot enterprise during the first implement of GMP system in China;
Marked the turning point of Gosun's quality system.

Ushered in the climax of development in the cephalosporin antibiotic market,
Gosun's products accounted for a large share of the domestic market,
The sales of major varieties such as ceftriaxone and cefotaxime were among the top.

Joining hands with the China National Inspection Institute,
improved the chemical structure of cefazolin sodium, which results a large clinical effect benifits.
The patented new drug cefazolin sodium pentahydrate (trade name: Xintailin®) was born.
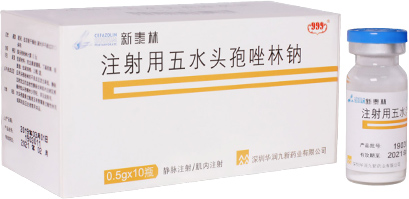
Xintailin® successfully launched as a new drug for its structural
technological innovation, and obtained 20-years patents
for both its structure and unique crystal form.
The drug became a blockbuster in China market afterwards.

Gosun was recognized as a national high-tech enterprise for the first time.

Gosun business both in domestic and overseas were boosting;
Shenyang Sanjiu factory started construction for the boosting business;
Gosun started EU certification project for powder injection workshop.

Shenyang Sanjiu factory was completed and put into production;
Xintailin became the top product of domestic antibiotic products.


Become the Rochefin CMO strategic partner for Roche,
Started the long-term partnership.
CR Gosun became the anti-infective division of CR Sanjiu;
New HQ building started construction;
CR Gosun entered a new and high-speed development era.

Acquired Zhejiang Zhongyi and enrich Gosun's product list to oral products.


License-in Ceftobiprole Medocaril Sodium for Injection from Basilea;
Co-develop Mitoxantrone Hydrochloride Injection for Tracing with SPU;
Awarded by the enterprise workstations of academicians and experts (provincial and municipal levels)

Founded a sales&marketing platform: China Resources Jiuzhong;
Founded an innovative drug incubation platform: China Resources Jiuchuang;
Gosun began to explore the perioperative treatment to pave the way for innovation and transformation.


